



The established technology behind Replexa+ is supported by a retrospective study of our patients, a chronic plantar fasciitis IRB study, and an in vitro scientific study.
Retrospective Patient Analysis
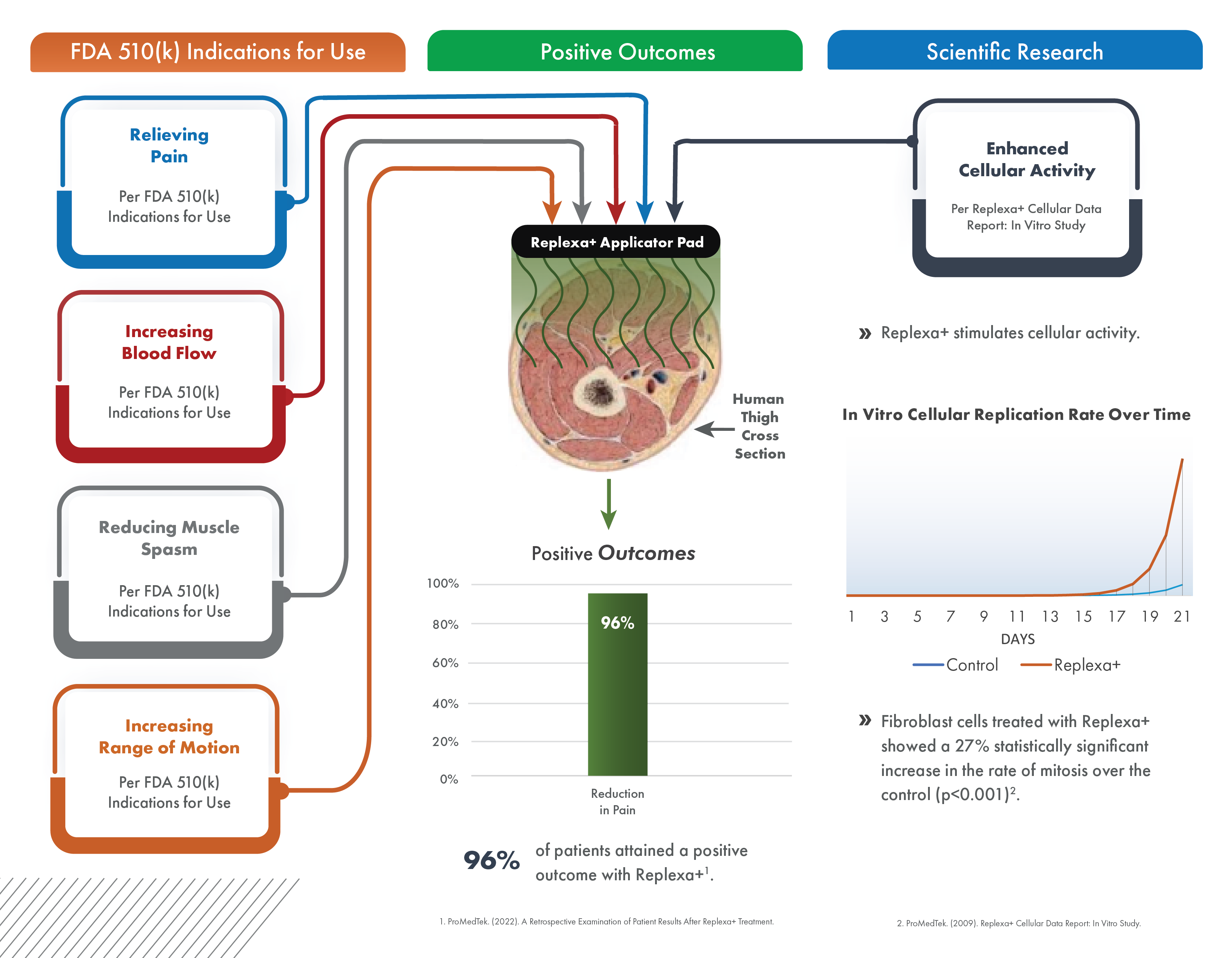
A retrospective analysis of patients who consistently used Replexa+ showed a 96% positive outcomes rate based on a reduction in pain and/or a relative improvement in quality of life.
Chronic Plantar Fasciitis IRB Study
In a chronic plantar fasciitis IRB study, 84% of patients attained a positive outcomes at a statistically significant (p<0.001).
Enhanced Cellular Activity
In an in vitro study, fibroblast cells treated with Replexa+ showed a statistically significant 27% increase in the rate of mitosis over the control (p<0.001).
In a chronic plantar fasciitis IRB study, patients attained a statistically significant (p<0.001) reduction in pain.
In vitro study of fibroblast cells treated with Replexa+ showed a statistically significant increase in the rate of mitosis over the control.
The FDA 510(k) clearance letter for Replexa+ that includes the indications for use.
The timeline for use varies from patient to patient, especially depending on the specific condition a patient is dealing with. Many patients begin to notice changes in a matter of weeks. Significant, lasting changes in the frequency and intensity of pain and symptoms may take a longer period of time to address.
Please visit the Contact Us page to request more information on how to order Replexa+ and to be connected with a local representative.
CONTRAINDICATIONS
Shortwave diathermy must not be applied over areas of the body that may contain metal (implants, surgical staples, etc.) for heat will become concentrated in that area increasing the possibility of tissue damage and deep burns.
Any patient with an implanted electronic device such as a cardiac pacemaker, bladder stimulator, spinal cord stimulator or electrodes for a myoelectric prosthesis, or implanted metallic leads must not be treated.
The following areas should not be treated with Replexa+:
1. Do not treat over the pelvic or low back area when an IUD is present.
2. Shortwave diathermy should not be applied over the pregnant or potentially pregnant uterus.
3. Shortwave diathermy should not be applied to the eye.
4. Do not apply over or in close proximity to active cancer (except in terminal / palliative / hospice care).
5. Shortwave diathermy should not be applied to the testes.
6. Do not treat tissues in individuals where the blood supply would be unable to follow the increase in metabolic demand.
7. Shortwave diathermy should not be applied over the epiphyseal areas (bone growth centers) of the bones of growing children.
8. Do not apply shortwave diathermy if there are tuberculous joints or acute infections within the treatment area.
WARNINGS
Always keep cables spaced apart. Keep cables at least several inches away from metal or grounded objects.
All equipment and accessories should be kept out of the reach of children or unqualified persons.
Do not apply over areas of hemorrhage or active bleeding.
Hearing aids should be removed during shortwave diathermy treatment.
Do not allow any liquids to penetrate the unit or its accessories while cleaning and disinfecting. Dry all sockets and connectors that have become wet before any further use.
PRECAUTIONS
Federal law restricts the sale of this device to, or on the order of a physician or any other practitioner licensed by law of the state in which he practices, to use or order the use of this device.
Use of controls or adjustments or performance of procedures other than those specified herein may result in hazardous exposure to radio frequency energy. Treatment should be administered only under the direct supervision of a health care professional.
Any bleeding tendency is increased by heating because of the increase in blood flow and vascularity of the heated tissues. Care, therefore, should be used in treating patients with therapeutic shortwave diathermy who have bleeding disorders.
Heating of the joint capsule in acute or subacute arthritis should be avoided.