

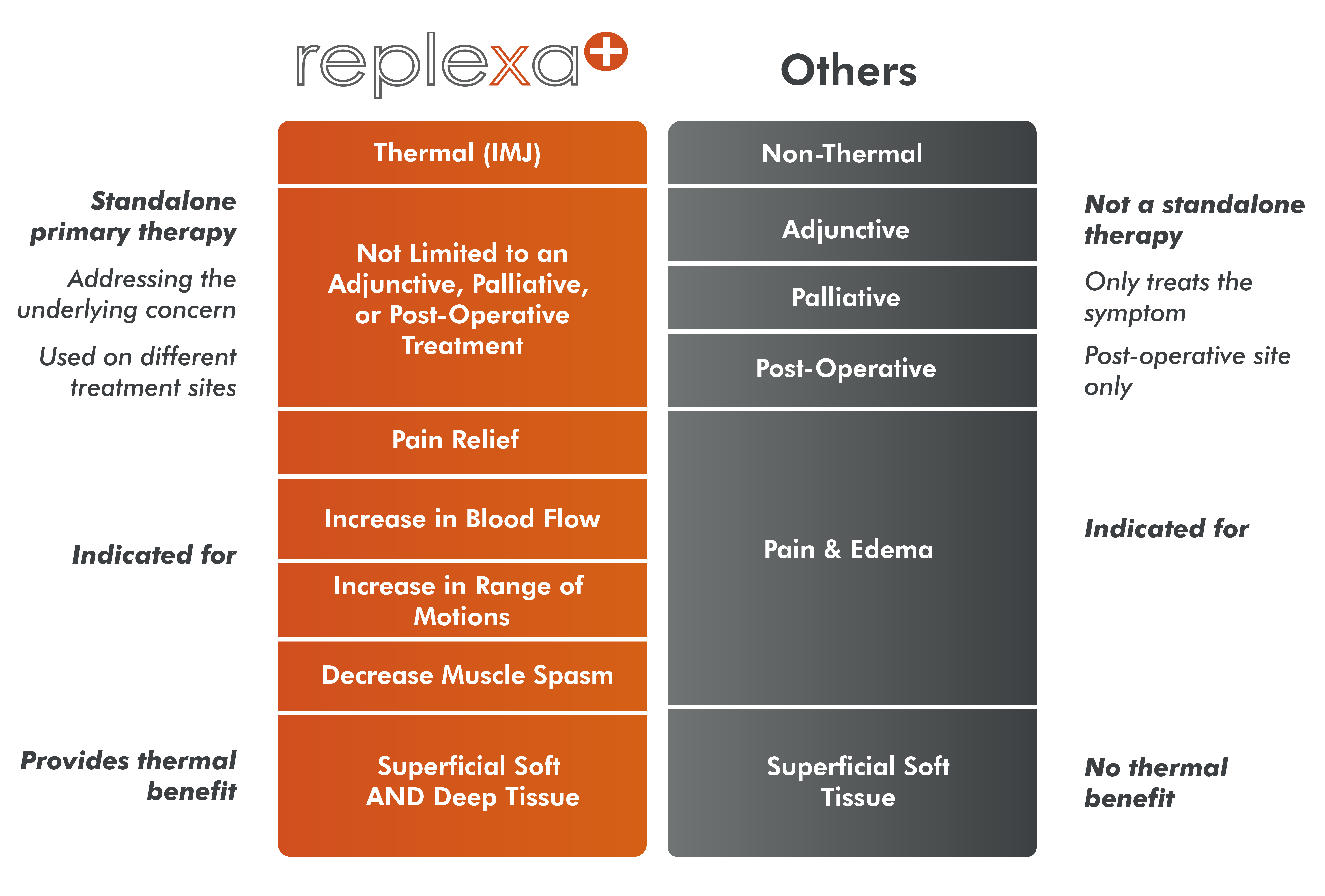
The FDA classifies shortwave diathermy into either a thermal or a non-thermal classification. The thermal classification can provide thermal clinical benefits while everything else only provides non-thermal benefits. The FDA has assigns different indications of use for thermal based on its proven safety and efficacy.
Non-thermal Shortwave Diathermy classified devices are indicated for adjunctive use in the palliative treatment of postoperative pain and edema in the superficial soft tissue.
Thermal Shortwave Diathermy classified devices provide a thermal benefit to the superficial soft and deep tissues, are not limited to an adjunctive, palliative, or post-operative treatment, and can be used as a standalone, primary therapy to treat various conditions.
The Replexa+ device is a thermal device with FDA indications for use that include relieving pain, increasing blood flow to tissues in the treatment area, reducing muscle spasm, increasing range of motion of contracted joints using heat and stretch techniques.

Replexa+ is a thermal shortwave diathermy device that the FDA has cleared to treat selected medical conditions such relieving pain, reducing muscle spasm, increasing range of motion, and increasing blood flow to tissues in the treatment area.
Various pain management modalities such as TENS units, PENS devices, and heating pads DO NOT share all of these indications for use.

Replexa+ is cleared by the FDA as a Class II, shortwave diathermy medical device available by prescription only.
The Replexa+ Shortwave Diathermy device delivers energy in the radio band of 27.12 MHz to provide deep heating therapeutic effects to body tissues. When shortwave diathermy is delivered to the body at intensities capable of generating a deep tissue temperature increase, it can be used to treat selected medical conditions such as:
1. Relieving pain
2. Reducing muscle spasm
3. Increasing range of motion of contracted joints using heat and stretch techniques.
4. Increasing blood flow to tissues in the treatment area.
A copy of the FDA 510(k) Clearance Letter is available for download here.
DOWNLOAD FDA 510(k) CLEARANCE LETTERYes. Replexa+ is a Class II medical device and is FDA cleared as safe and effective.
No, the applicators do not need to touch the skin of the treatment area to work. The energy comes out of the applicator and will be able to deliver the therapy to the treatment area through clothing.
Most patients will not feel a notable sensation during Replexa+ treatments. Replexa+ shortwave diathermy is applied in short, high-energy pulses. As a result, the depth of penetration is increased while having a positive effect on relieving pain, reducing muscle spasm, increasing range of motion, and increasing blood flow to tissues in the treatment area with little to no sensation felt on the skin.
The timeline for noticing a difference varies from patient to patient, especially depending on the specific condition a patient is being treated for. Many patients begin to notice changes in a matter of weeks. Significant, lasting changes in the frequency and intensity of their pain and symptoms may take a longer period of time address.